Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Astrochemistry
Brett McGuire
Interstellar explorer is expanding our molecular knowledge of space
by Sam Lemonick
August 14, 2020
| A version of this story appeared in
Volume 98, Issue 31

Credit: Courtesy of Brett McGuire (McGuire); European Southern Observatory (Taurus Molecular Cloud); Molview (structures); Shutterstock (radio telescope)
Chemists know a lot about how atoms and molecules behave—lugging around any chemistry textbook will tell you that. But almost all of that knowledge is about how chemistry happens here on Earth, and the conditions in the vast majority of the universe are very, very different.
Brett McGuire, a newly minted chemistry professor at the Massachusetts Institute of Technology, is trying to figure out the rules of chemistry in outer space, specifically in the dark, cold dust clouds where planets and stars are born.
Advertisement
That would be a lot easier if we could visit these places, but they’re light-years away. Instead, he and his collaborators combine chemistry and astronomy techniques to identify molecules in these regions. First, McGuire makes molecules in the lab he thinks might be found in outer space, then carefully measures the unique signature of light they absorb. Next, using massive telescopes, McGuire and his colleagues search for those spectroscopic fingerprints in the radiation shining out from these clouds. By identifying molecules, McGuire hopes he and others will be able to piece together what kind of chemistry can happen in space.
“Our job, with only photons from the sky, is to figure out what molecules are there, where they came from, and where they go next,” he says.
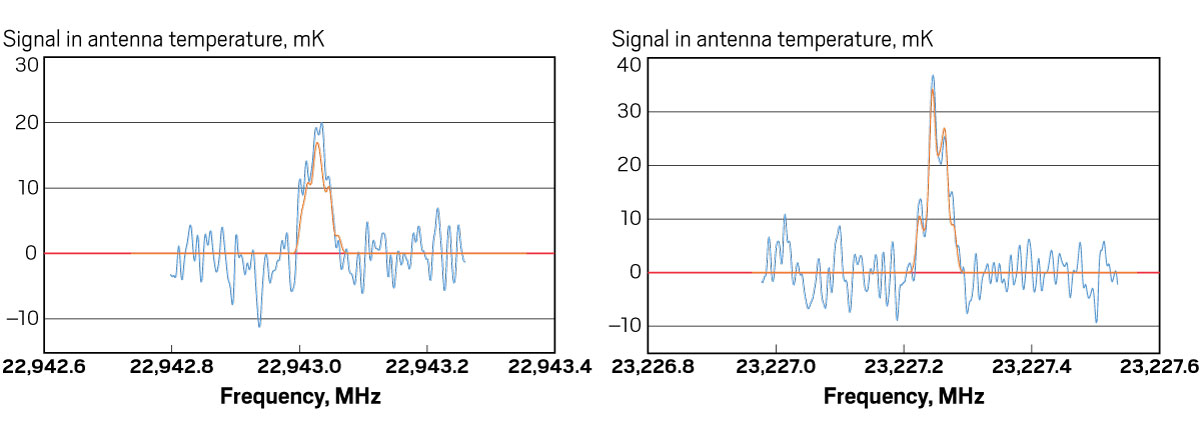
So far, McGuire is doing a good job of figuring out what’s there. “He’s one of the most successful new interstellar molecule hunters,” says Ewine van Dishoeck, an astrochemist at Leiden Observatory and one of McGuire’s collaborators. In 2016, McGuire and colleagues identified the first chiral molecule in space, propylene oxide, and in 2018 he helped identify the first aromatic molecule, benzonitrile. Astronomers knew chiral and aromatic molecules existed in interstellar space, but no one had determined which ones, exactly, were out there.
McGuire thinks being both an astronomer and a chemist gives him an edge. “I speak both languages,” he says, and thus he can build teams with experts from both fields. “That’s why we’ve been successful.”
As he prepares to start his own research group at MIT this fall, McGuire is pushing deeper into the unknown. Rather than starting with molecules he thinks are out there, like propylene oxide and benzonitrile, McGuire is letting chemistry guide his search. By performing reactions in the lab that might happen in interstellar dust clouds, his team makes molecules that have never been seen on Earth. Then, they go look for them in telescope data. The first results using this approach will be published later this year.
For McGuire, this is just the beginning of understanding interstellar chemistry, a process he thinks could take decades. Learning the identity and location of new aromatic rings could change scientists’ theories about how stars and planets form. Identifying chiral molecules could help chemists figure out why biological molecules like DNA tend to twist the same direction.
“I wouldn’t say we’re beginning to have answers,” McGuire says. “I’d say we’re beginning to be able to ask the right questions.”
Vitals
Current affiliation: Massachusetts Institute of Technology
Age: 33
PhD alma mater: California Institute of Technology
Hometown: Charleston, Illinois
If I weren’t a chemist, I’d be: A geologist, “or something similar that could keep me outdoors”
If I were an element, I’d be: Tin. “It’s an underrated metal both allomantically and feruchemically!”
Chemists know a lot about how atoms and molecules behave—lugging around any chemistry textbook will tell you that. But almost all of that knowledge is about how chemistry happens here on Earth, and the conditions in the vast majority of the universe are very, very different.
Vitals
▸ Current affiliation: Massachusetts Institute of Technology
▸ Age: 33
▸ PhD alma mater: California Institute of Technology
▸ Hometown: Charleston, Illinois
▸ If I weren’t a chemist, I’d be: A geologist, “or something similar that could keep me outdoors”
▸ If I were an element, I’d be: Tin. “It’s an underrated metal both allomantically and feruchemically!”
Brett McGuire, a newly minted chemistry professor at the Massachusetts Institute of Technology, is trying to figure out the rules of chemistry in outer space, specifically in the dark, cold dust clouds where planets and stars are born.
That would be a lot easier if we could visit these places, but they’re light-years away. Instead, he and his collaborators combine chemistry and astronomy techniques to identify molecules in these regions. First, McGuire makes molecules in the lab he thinks might be found in outer space, then carefully measures the unique signature of light they absorb. Next, using massive telescopes, McGuire and his colleagues search for those spectroscopic fingerprints in the radiation shining out from these clouds. By identifying molecules, McGuire hopes he and others will be able to piece together what kind of chemistry can happen in space.
“Our job, with only photons from the sky, is to figure out what molecules are there, where they came from, and where they go next,” he says.
So far, McGuire is doing a good job of figuring out what’s there. “He’s one of the most successful new interstellar molecule hunters,” says Ewine van Dishoeck, an astrochemist at Leiden Observatory and one of McGuire’s collaborators. In 2016, McGuire and colleagues identified the first chiral molecule in space, propylene oxide, and in 2018 he helped identify the first aromatic molecule, benzonitrile. Astronomers knew chiral and aromatic molecules existed in interstellar space, but no one had determined which ones, exactly, were out there.
McGuire thinks being both an astronomer and a chemist gives him an edge. “I speak both languages,” he says, and thus he can build teams with experts from both fields. “That’s why we’ve been successful.”
As he prepares to start his own research group at MIT this fall, McGuire is pushing deeper into the unknown. Rather than starting with molecules he thinks are out there, like propylene oxide and benzonitrile, McGuire is letting chemistry guide his search. By performing reactions in the lab that might happen in interstellar dust clouds, his team makes molecules that have never been seen on Earth. Then, they go look for them in telescope data. The first results using this approach will be published later this year.
For McGuire, this is just the beginning of understanding interstellar chemistry, a process he thinks could take decades. Learning the identity and location of new aromatic rings could change scientists’ theories about how stars and planets form. Identifying chiral molecules could help chemists figure out why biological molecules like DNA tend to twist the same direction.
“I wouldn’t say we’re beginning to have answers,” McGuire says. “I’d say we’re beginning to be able to ask the right questions.”




















Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter