Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.

Imaging
Chemistry’s most powerful tools
Modern chemistry depends on analytical instruments. From the micro end of the spectrum—where researchers study reactions one molecule at a time—to the macro—where engineers run industrial reactors that crank out tons of product—the information that these tools provide enables scientists to track molecules and understand their behavior
by Mitch Jacoby
August 11, 2023
| A version of this story appeared in
Volume 101, Issue 26
Nuclear magnetic resonance spectroscopy and imaging

Josef Dadok (right) and coworkers at the Institute of Scientific Instruments in the former Czechoslovakia discuss nuclear magnetic resonance data in this photo from 1960.
Credit: Institute of Scientific Instruments
Few analytical methods have advanced the practice of chemistry and medicine as much as nuclear magnetic resonance (NMR) spectroscopy and its imaging offshoot, magnetic resonance imaging (MRI). These techniques, which rely on interactions between atomic nuclei and magnetic fields, provide researchers with indispensable tools for determining the structures of molecules in solids, liquids, and gases and for imaging subtle details of tissues and internal organs. Early commercial NMR instruments enabled chemists in the 1950s to analyze relatively small organic molecules, but the size and molecular complexity of compounds analyzed and identified by NMR methods grew rapidly. Researchers today routinely use these instruments to study RNA, DNA, other types of macromolecules, and large inorganic complexes. And they depend on NMR-based methods to continue advancing structural biology, medicine, and materials science.
X-ray crystallography
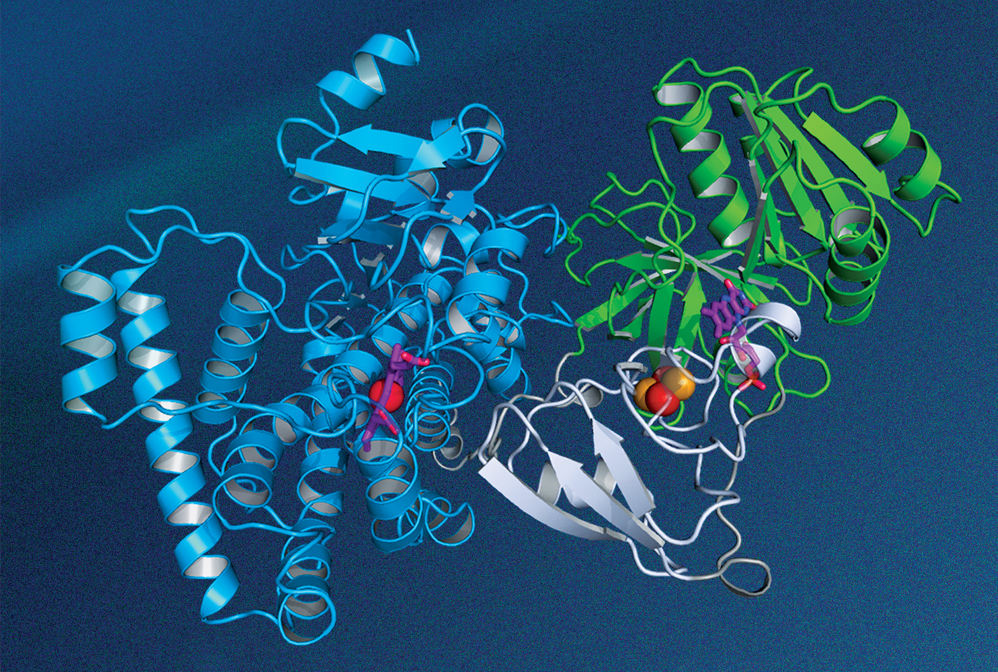
The structure of this P450 enzyme, which is made by a thermophilic bacterium, was determined by researchers at Hubei University using X-ray crystallography.
Credit: Rey-Ting Guo/Chun-Chi Chen
In the early 20th century, scientists discovered that irradiating a crystal with a beam of X-rays causes the beam to be scattered into many well-defined directions. Father and son physicists William Henry Bragg and William Lawrence Bragg worked out the mathematical theory from which the atomic structure of a crystal could be deduced from the observed scattering patterns. Their work, for which they shared the 1915 Nobel Prize in Physics, remains the cornerstone of modern X-ray crystallography. In recent years, researchers have used the technique to determine the 3D geometry of complex biological structures, such as a P450 enzyme, which plays a central role in cellular drug metabolism.
Mass spectrometry
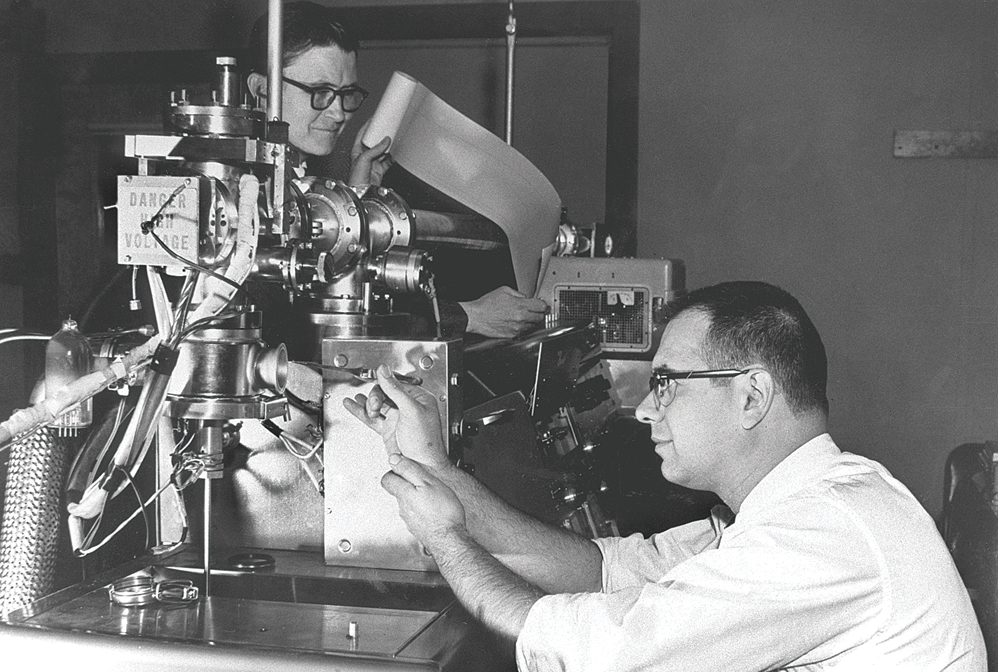
Fred McLafferty (back) and Roland Gohlke work on a mass spectrometer at Dow Chemical circa 1960.
Credit: Dow Chemical
The mass of an atom, molecule, or microscopic particle is one of its most fundamental properties and has long been used to identify or confirm the presence of these species. Some of the earliest work in detecting atomic masses was conducted in the 1910s by British physicist J. J. Thomson and his assistant Francis William Aston, who built instruments that used electric and magnetic fields to deflect streams of ions. Their work led to the discovery of atomic isotopes. In the decades that followed, researchers made a staggering number of innovations, greatly increasing these instruments’ mass resolution and detection sensitivity, broadening their ionization and sample-inlet capabilities, and extending the range of applications in which they are used. Some of today’s mass specs are used for single-cell and single-molecule mass measurements, capabilities that can advance biology and medicine.
Chromatography
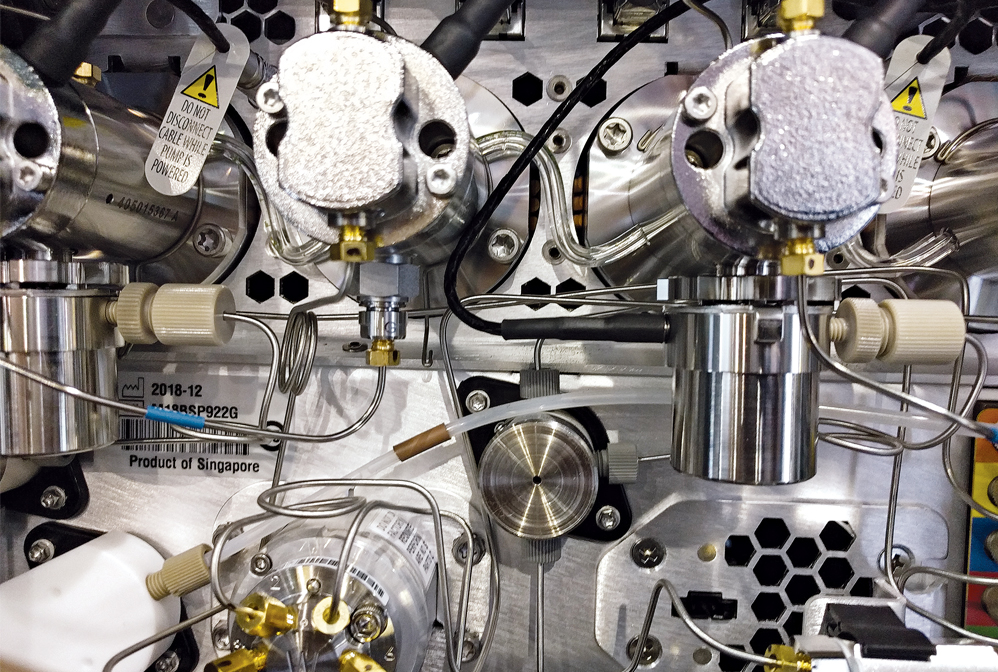
The pumping network lies at the heart of modern high-performance liquid chromatography instruments.
Credit: Marc Reisch/C&EN
Mixing chemicals is relatively easy. Separating them is a challenge, one that long ago led scientists to develop chromatography methods for isolating the components of chemical mixtures. Researchers use these techniques to, for example, identify unknown substances, evaluate the purity of valuable compounds, and assess the selectivity of synthesis methods. Some of the earliest work focused on separating and purifying naturally occurring blends of plant pigments by exploiting differences in the strength with which component molecules bind to particles in a separation column. One hundred years of innovation brought only modest changes to separation fundamentals but extraordinary changes to instrumentation. Around 1950, fully manual methods gave way to powered systems for gas chromatography, a technique for separating volatile compounds. High-performance liquid chromatography caught on in the 1970s, eventually providing ways to separate and analyze a very broad range of compounds, including chiral pharmaceutical agents.
Microscopy and molecular imaging
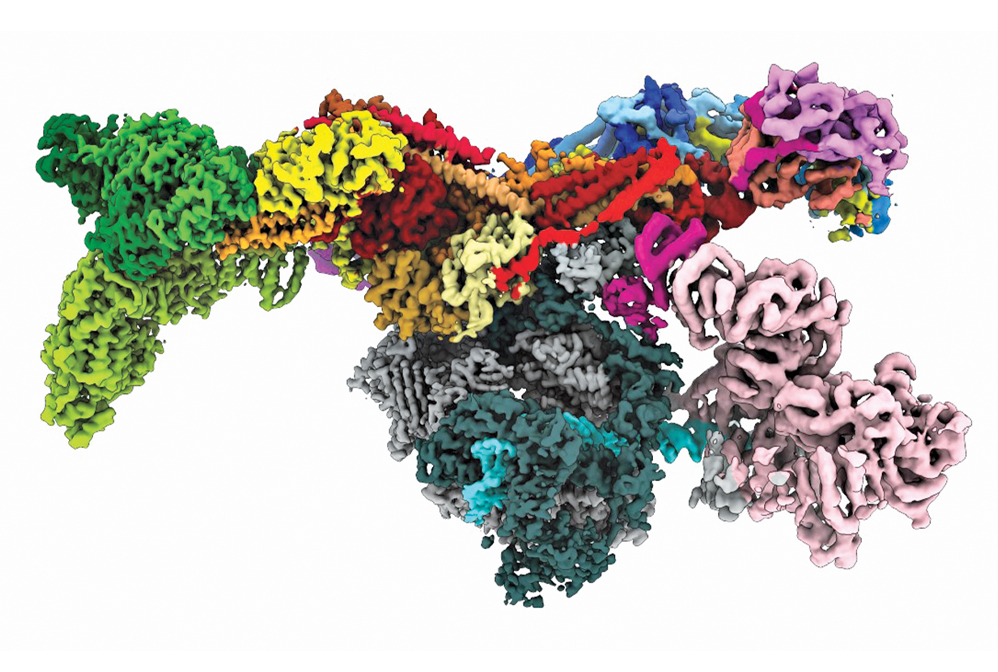
Aided by recent advances in electron microscopy, researchers deduced the structure of the human preinitiation complex.
Credit: Yuan He/Science
For hundreds of years, people curious about nature used magnifying glasses to reveal structures too small to be seen with the naked eye. In the past century, scientists have radically advanced those capabilities, designing instruments that provide unprecedented views of atoms and molecules. Field-ion microscopes and transmission electron microscopes led the way in 1955 and 1970, respectively, generating images in which each dot corresponded to a single atom in a metal specimen. Scanning probe microscopes, which followed in the 1980s, enabled researchers to not only image atoms but also manipulate them one at a time and assemble and study nanostructures. Scientists continue to innovate and devise methods for imaging increasingly complex molecular structures, such as the human preinitiation complex, shown here. This cellular machine transcribes genes and holds the keys to treating many diseases.
Ultrafast diffraction for tracking molecular dynamics
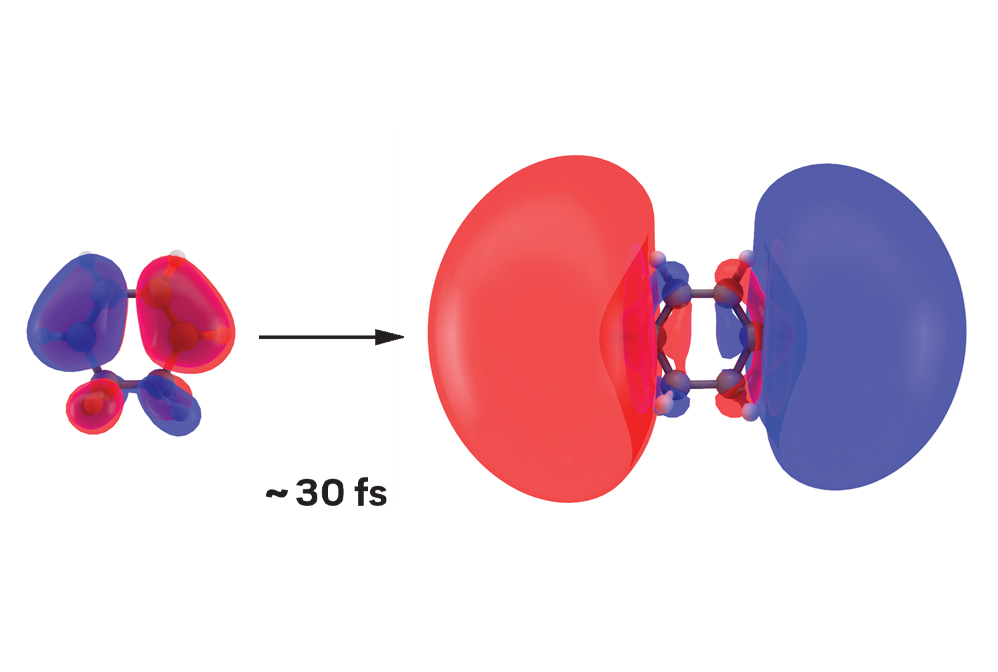
When 1,3-cyclohexadiene absorbs ultraviolet light, it undergoes instantaneous excitation. The light pulse triggers the first step of a chemical reaction by causing a ground-state orbital (left) to balloon into an excited one. How long is instantaneous? Just 30 fs.
Credit: Greg Stewart/SLAC National Accelerator Laboratory
Making and breaking chemical bonds are the basic steps of chemical reactions. But because those erratic motions of electrons and nuclei typically occur within a few femtoseconds—or millionths of a billionth of a second—tracking them is no mean feat. Yet in the past decade, researchers have devised methods to record stop-action photographs of these fleeting molecular dance moves. These methods use X-ray free-electron lasers for ultrafast diffraction of X-rays and electron cameras for ultrafast diffraction of electrons. Scientists can stitch these images together to construct movies of chemical reaction dynamics. These movies reveal the elemental steps that guide a reactant to form one or more products.






Nuclear magnetic resonance spectroscopy and imaging

Few analytical methods have advanced the practice of chemistry and medicine as much as nuclear magnetic resonance (NMR) spectroscopy and its imaging offshoot, magnetic resonance imaging (MRI). These techniques, which rely on interactions between atomic nuclei and magnetic fields, provide researchers with indispensable tools for determining the structures of molecules in solids, liquids, and gases and for imaging subtle details of tissues and internal organs. Early commercial NMR instruments enabled chemists in the 1950s to analyze relatively small organic molecules, but the size and molecular complexity of compounds analyzed and identified by NMR methods grew rapidly. Researchers today routinely use these instruments to study RNA, DNA, other types of macromolecules, and large inorganic complexes. And they depend on NMR-based methods to continue advancing structural biology, medicine, and materials science.
X-ray crystallography

In the early 20th century, scientists discovered that irradiating a crystal with a beam of X-rays causes the beam to be scattered into many well-defined directions. Father and son physicists William Henry Bragg and William Lawrence Bragg worked out the mathematical theory from which the atomic structure of a crystal could be deduced from the observed scattering patterns. Their work, for which they shared the 1915 Nobel Prize in Physics, remains the cornerstone of modern X-ray crystallography. In recent years, researchers have used the technique to determine the 3D geometry of complex biological structures, such as a P450 enzyme, which plays a central role in cellular drug metabolism.
Mass spectrometry

The mass of an atom, molecule, or microscopic particle is one of its most fundamental properties and has long been used to identify or confirm the presence of these species. Some of the earliest work in detecting atomic masses was conducted in the 1910s by British physicist J. J. Thomson and his assistant Francis William Aston, who built instruments that used electric and magnetic fields to deflect streams of ions. Their work led to the discovery of atomic isotopes. In the decades that followed, researchers made a staggering number of innovations, greatly increasing these instruments’ mass resolution and detection sensitivity, broadening their ionization and sample-inlet capabilities, and extending the range of applications in which they are used. Some of today’s mass specs are used for single-cell and single-molecule mass measurements, capabilities that can advance biology and medicine.
Chromatography

Mixing chemicals is relatively easy. Separating them is a challenge, one that long ago led scientists to develop chromatography methods for isolating the components of chemical mixtures. Researchers use these techniques to, for example, identify unknown substances, evaluate the purity of valuable compounds, and assess the selectivity of synthesis methods. Some of the earliest work focused on separating and purifying naturally occurring blends of plant pigments by exploiting differences in the strength with which component molecules bind to particles in a separation column. One hundred years of innovation brought only modest changes to separation fundamentals but extraordinary changes to instrumentation. Around 1950, fully manual methods gave way to powered systems for gas chromatography, a technique for separating volatile compounds. High-performance liquid chromatography caught on in the 1970s, eventually providing ways to separate and analyze a very broad range of compounds, including chiral pharmaceutical agents.
Microscopy and molecular imaging

For hundreds of years, people curious about nature used magnifying glasses to reveal structures too small to be seen with the naked eye. In the past century, scientists have radically advanced those capabilities, designing instruments that provide unprecedented views of atoms and molecules. Field-ion microscopes and transmission electron microscopes led the way in 1955 and 1970, respectively, generating images in which each dot corresponded to a single atom in a metal specimen. Scanning probe microscopes, which followed in the 1980s, enabled researchers to not only image atoms but also manipulate them one at a time and assemble and study nanostructures. Scientists continue to innovate and devise methods for imaging increasingly complex molecular structures, such as the human preinitiation complex, shown here. This cellular machine transcribes genes and holds the keys to treating many diseases.
Ultrafast diffraction for tracking molecular dynamics

Making and breaking chemical bonds are the basic steps of chemical reactions. But because those erratic motions of electrons and nuclei typically occur within a few femtoseconds—or millionths of a billionth of a second—tracking them is no mean feat. Yet in the past decade, researchers have devised methods to record stop-action photographs of these fleeting molecular dance moves. These methods use X-ray free-electron lasers for ultrafast diffraction of X-rays and electron cameras for ultrafast diffraction of electrons. Scientists can stitch these images together to construct movies of chemical reaction dynamics. These movies reveal the elemental steps that guide a reactant to form one or more products.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter