Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
C&EN’s Molecules Of The Year
Structure Menagerie: Compounds that made headlines in 2015
December 21, 2015
| A version of this story appeared in
Volume 93, Issue 49
Hexafluorocyclohexane Sets A Polarity Record
Chemists this year made the all-cis isomer of 1,2,3,4,5,6-hexafluorocyclohexane, a crystalline ring molecule with all six fluorines pointing “up” and all six hydrogens pointing “down.” This compound, produced by David O’Hagan, Neil S. Keddie, and coworkers of the University of St. Andrews, is remarkable for having a dipole moment of 6.2 debyes, making it the most polar nonionic aliphatic compound known to exist (Nat. Chem. 2015, DOI: 10.1038/nchem.2232). The deceptively simple molecule was a complex synthetic target because it required 12 steps to make and has nine possible isomers that can adopt up to 15 conformations.

Giant Porphyrin Goes Big On Aromaticity
By increasing the number of pyrrole groups in a porphyrin ring from the usual four to 12, a team led by Dongho Kim of Yonsei University and Atsuhiro Osuka of Kyoto University succeeded in making a dodecaphyrin containing 50 π electrons—the largest aromatic molecule known to date (Chem. Eur. J. 2015, DOI: 10.1002/chem.201500650). The compound bests the former aromaticity record holder, a [46π]decaphyrin the same researchers made last year.
[5]Radialene Fills In A Missing Hydrocarbon Gap
Radialenes are a unique class of star-shaped cyclic hydrocarbons that have double bonds radiating out from each carbon in the ring. Prior to this year, chemists had made the three-, four-, and six-membered ring versions of these polyenes, but never [5]radialene—it had always been too unstable. A research team including Emily G. Mackay and Michael S. Sherburn of Australian National University and Michael N. Paddon-Row of the University of New South Wales developed a synthetic strategy to make the missing link for the first time (J. Am. Chem. Soc. 2015, DOI: 10.1021/jacs.5b07445).
Cobalt-Boron Molecular Drum Beats Coordination Record

A multinational research team this year created a drum-shaped cobalt-boron species in the gas phase, CoB16–, which set a record for highest coordination number in a molecule. The sandwich complex created by Alexander I. Boldyrev of Utah State University, Lai-Sheng Wang of Brown University, and their colleagues consists of two B8 rings connected to the central cobalt atom via 16 bonds, the theoretical maximum based on the number of available atomic orbitals (Nat. Commun. 2015, DOI: 10.1038/ncomms9654). The researchers made the molecular drum by vaporizing a cobalt-boron target with a laser and then isolating CoB16– clusters from the product mixture and analyzing them spectroscopically and computationally.
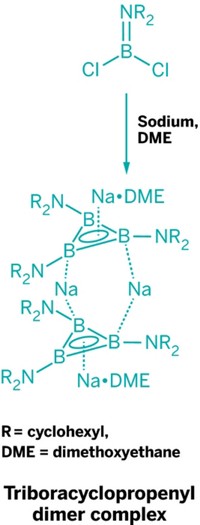
Boron Forms A Featherweight Aromatic Compound
By virtue of synthesizing a stable B3 ring, inorganic chemists prepared the lightest aromatic species that is experimentally possible this year. The sandwich molecule made by Thomas Kupfer, Holger Braunschweig, and Krzysztof Radacki of Julius Maximilian University Würzburg contains two triboracyclopropenyl dication rings connected by sodium ions. The new species could serve a practical purpose as the first of a family of precursor compounds for preparing semiconducting, superconducting, and magnetic materials (Angew. Chem. Int. Ed. 2015, DOI: 10.1002/anie.201508670). The Würzburg team showed through computational, spectroscopic, and electrochemical studies that the B3 ring has an electronic structure consistent with classical aromatic carbon compounds, such as the cyclopropenyl cation and benzene.
Superstrong Organic Reducing Agent

Organic electron donors with exceptionally negative redox potentials have emerged as useful reagents in organic synthesis to complement traditional metal-based reducing agents. Chemists led by John A. Murphy of the University of Strathclyde and C. Adam Dyker of the University of New Brunswick pushed this class of compounds to a new level by creating a tetra(iminophosphorano)-substituted bispyridinylidene that has a record redox potential of –1.70 V, surpassing the previous record by about 0.2 V (Angew. Chem. Int. Ed. 2015, DOI: 10.1002/anie.201505378). The two-electron donor molecule, which can be used in multigram-scale reactions, is the first reagent strong enough to reduce recalcitrant malononitriles (shown) and sulfonamides without light-driven photoexcitation.
C&EN's YEAR IN REVIEW
Top Headlines of 2015
- Chemical Makers Looked To Big Deals
- NASA Got Up Close And Personal With Pluto
- Opposition To Neonicotinoids Intensified
- 2015 Nobel Prizes In Science At A Glance
- Climate Pact Clinched
- Little Good News For Chemistry Job Outlook In 2015
- Pfizer To Merge Again, This Time With Allergan
- Oil And Gas Industry Under Pressure
- Greening Up Fracking
- An Industry In Spin Cycle
- Finally, Emoji For Chemists
- A Big Deal For Chemists
- Tianjin Explosion Put Spotlight On Safety
- Women Assumed Leadership Roles At American Chemical Society
- Artificial Ingredients In The Crosshairs
- Jacqueline K. Barton, Unwavering Chemistry Champion
- House Science Committee Chair Pummeled Science Agencies
- NIST Veteran Became U.S. Government's Top Chemist
- Gene-Editing Technique Raised Ethics Questions
- World Chemical Production At A Glance
- Classroom Fires During Science Demonstrations Spark Concern
- Climate Was Right For Deal-Making
- American Chemical Society Dives Deeper Into Open Access With The Debut Of ACS Omega
- Lego Began Research On Switching To A Biobased Plastic
- New Chair Took The Helm At Troubled Chemical Safety Board
- Genetically Modified Foods In The Spotlight
- Overhaul Of U.S. Chemical Law Moved
- ACS Scholars Program Turned 20
- American Chemical Society Expanded Its Global Reach
- Scientists Called For Standardized Antibodies
Top Research of 2015
- Flexible Electronics You Can Inject
- Special Delivery For Sensitive Reagents
- Yeast Programmed For Opioid Total Synthesis
- Miracle Machine Builds Molecules On Demand
- Nickel Shines As A Catalyst
- 3-D Printing Takes On A New Dimension
- Atomically Thin Films Grow In Number
- Digging In The Dirt Yields Novel Bacteria Fighter
- Keeping GMOs On A Leash
- A Liquid With Holes In It
- Electron Microscopy Provides Unprecedented Close-ups
Revisiting Research of 2005


![Structure of the large porphyrin molecule [50π]Dodecaphyrin. Structure of the large porphyrin molecule [50π]Dodecaphyrin.](https://s7d1.scene7.com/is/image/CENODS/09349-cover13-MSAstruc-690?$responsive$&wid=700&qlt=90,0&resMode=sharp2)



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter