Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Research of the year
C&EN reviews notable chemistry research advances from 2017
December 18, 2017 | Volume 95 Issue 49
Computer-driven research reached new milestones
Machine learning and quantum computing tackled complex problems
By Mitch Jacoby
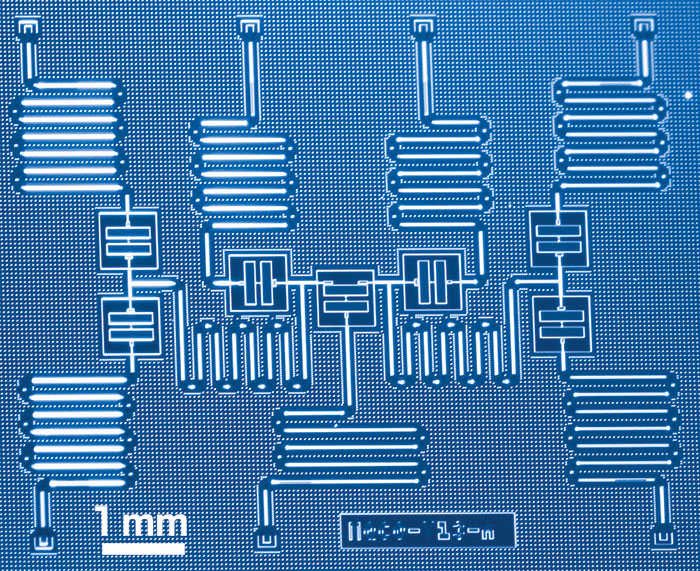

Computers have been powering scientific discovery for decades. But this year, several developments in machine learning and quantum computing raised the bar substantially.
As academic researchers exploited the advances to tackle problems in basic science, chemical companies including BASF and Dow Chemical formed alliances with computer giants such as IBM and Hewlett Packard Enterprise to capitalize on the advances for commercial applications.
Machine learning refers to algorithms that enable computers to go beyond rigid programming instructions and “learn” from and implement decisions and make predictions on the basis of large sets of data. Programs for voice and face recognition, spam email filtration, and weather forecasting use such algorithms.
On the chemistry front, David Baker of the University of Washington and coworkers reported using this technique to determine the three-dimensional conformations of 600 families of proteins for which structures had been unknown (Science 2017, DOI: 10.1126/science.aah4043). And an international team used the method to enable computers to predict a compound’s scent on the basis of its molecular structure. That advance broadens understanding of olfaction and one day may benefit the fragrance industry (Science 2017, DOI: 10.1126/science.aal2014). Machine-learning strategies also made headway in calculating molecular electronic structures in a manner that bypasses the most computationally intense aspects of density functional theory (Nat. Commun. 2017, DOI: 10.1038/s41467-017-00839-3).

As some researchers pushed the chemistry-predicting capabilities of traditional computers, scientists at IBM, Microsoft, and Google pushed forward with quantum computers. Unlike conventional machines, which use transistors and memory cells to process ones and zeros that approximate electron wave functions, quantum computers use magnetic elements or other types of two-state quantum systems, known as qubits, to represent wave functions as superpositions of electron energies and states.
That strategy should enable quantum computers to calculate properties of complex molecules that conventional computers are not powerful enough to address. This feat has not yet been demonstrated. Nonetheless, researchers at IBM reached a milestone this year by using a 7-qubit quantum computer to calculate the ground-state energies of lithium hydride and beryllium hydride. These were the first calculations of molecules containing atoms larger than hydrogen and helium using quantum computing (Nature 2017, DOI: 10.1038/nature23879).
Electrosynthesis got chemists charged up
Using electric current as a reagent streamlined organic reactions by avoiding hazardous chemicals, generating less waste, and reducing cost
By Steve Ritter


When it comes to electrochemistry, the first things that pop into mind might be batteries and solar cells, or industrial processes like electroplating. Organic synthesis is not usually part of the conversation. Although electrochemical synthesis is well established, chemists have been reluctant to adopt the approach for common organic reactions, such as C–H activation or arene cross-couplings, believing it is too cumbersome or expensive. But the technology has been trending since 2015 and fully emerged this year.
“Synthetic chemists have long viewed electrochemistry as an area where a few people do interesting reactions that are difficult for everyone else to repeat,” commented Kevin D. Moeller of Washington University in St. Louis. “That view is changing: The field is undergoing a dramatic uptick in popularity.”
Electric current, when used as a surrogate reagent, offers the ability to avoid toxic or dangerous reagents, protecting groups, and catalysts typically used in organic synthesis. Moreover, electrosynthesis can reduce or eliminate the need to heat and cool reaction vessels, cutting energy consumption. Those advantages play right into the hands of today’s synthetic organic chemist, who is faced with the challenge of creating increasingly complex molecules, but doing so in a greener, more sustainable, safer, and more cost-effective manner than before.
In one example, Siegfried R. Waldvogel of Johannes Gutenberg University Mainz and his group in collaboration with researchers at Evonik Industries created a one-step electrochemical protocol for cross-coupling reactions to make biaryl diols and diamines, which are important chemical intermediates (C&EN, March 13, page 23).
“This stuff is extraordinary,” Waldvogel told C&EN. “Electrosynthesis represents a disruptive technology and will be a game changer for industry.”
Besides pushing to develop new electrochemically enabled reactions, academic research groups are helping develop user-friendly instrumentation specifically for the organic synthesis community. Last year, Waldvogel helped launch IKA’s lab-scale continuous-flow electrosynthesis system, called ElectraSyn Flow. And this year, Phil S. Baran of Scripps Research Institute California, whose group is turning more often to electrosynthesis for difficult steps in preparing terpene natural products, helped IKA launch ElectraSyn 2.0, a modernized version of the company’s electrochemical synthesis system.
“This is incredibly impactful research,” Baran said. “The reemergence of electrochemistry deserves our attention.”
Modified and newly discovered enzymes sparked innovative chemistry
Advances this year included improved alkene oxidations, fatty acid decarboxylations, and aromatic alkylations
By Stu Borman
Enzymes play a key role in chemistry research and in the chemical and food processing industries. Evidence comes from the pages of C&EN: A search shows that enzymes were covered in about 200 articles this year, roughly four articles per issue. Among those were many tales of cutting-edge discoveries. For example, researchers hunted down new enzymes capable of catalyzing syntheses useful to the biotech industry, and they artificially evolved known enzymes to orchestrate tough organic reactions. “I believe we are entering a new era of enzyme discovery,” says Emily P. Balskus of Harvard University. “Expanding the types of molecules we can access using biocatalysis and synthetic biology requires that we identify enzymes that perform new chemical transformations, either through discovery or engineering.” Here are three of C&EN’s favorite enzyme feats of 2017.
Friedel-Crafty bacteria
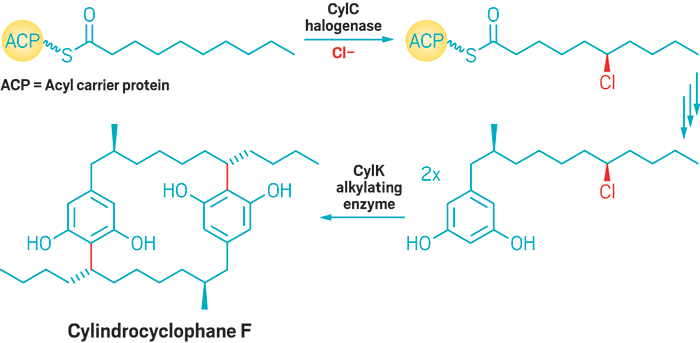

Balskus’s group at Harvard pinpointed the enzymes that cyanobacteria use to synthesize aromatic natural products called cylindrocyclophanes. In 1877, Charles Friedel and James Crafts discovered that a Lewis acid could add an alkyl halide’s alkyl group to an aromatic ring. Balskus and coworkers showed that cyanobacteria developed the same type of reaction first (Nat. Chem. Biol. 2017, DOI: 10.1038/nchembio.2421). One enzyme they found, CylK, decorates aromatic rings with alkyl groups from alkyl halides. Another, CylC, generates the alkyl chlorides that the cyanobacteria need to run the CylK reaction (shown in scheme). The researchers are currently trying to solve CylK’s crystal structure and hope to engineer the enzyme to accept a wider variety of building blocks as substrates.
Evolved oxidizer
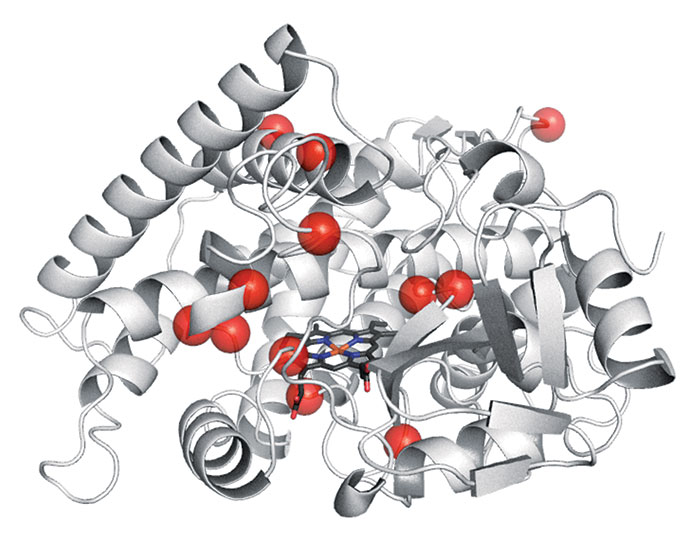

To oxidize the end carbon of a terminal alkene to form an aldehyde—an anti-Markovnikov alkene oxidation—chemists typically have had to use nonenantioselective and fairly unproductive methods. Frances H. Arnold of Caltech and coworkers employed a technique called directed evolution to develop an anti-Markovnikov enzyme that works better. Directed evolution is an iterative protein-mutation and screening process that endows enzymes with abilities they weren’t born with. Arnold’s team identified 12 amino acid substitutions (red circles) that convert a conventional alkene oxidation enzyme called P450LA1 into a predominantly stereoselective anti-Markovnikov catalyst called aMOx (Science 2017, DOI: 10.1126/science.aao1482). The productivity of aMOx (heme group is black, blue, and red stick structure) is nearly 400 times that of some existing commercial alkene oxidation catalysts.
Enlightening discovery
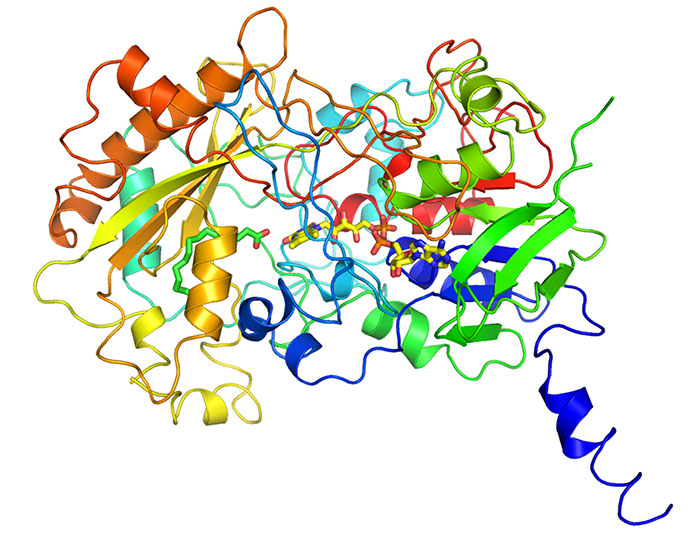

A team led by Fred Beisson of the French Alternative Energies & Atomic Energy Commission found that microalgae possess an unusual enzyme that harnesses light to decarboxylate fatty acids to alkanes or alkenes (Science 2017, DOI: 10.1126/science.aan6349). The researchers investigated microalgae that synthesize hydrocarbons but don’t have genes for the enzymes that typically perform that task. By purifying enzymes from the microalgae, they identified fatty acid photodecarboxylase (shown with the cofactor flavin adenine dinucleotide and a palmitic acid substrate as stick structures), a photoenzyme with unprecedented activity: converting fatty acids into 14- to 18-carbon alkanes or alkenes. The new photoenzyme could be useful for producing biobased hydrocarbons.
Flow chemistry advanced in industry
Lilly chemists capitalized on continuous chemical manufacturing to make a chemotherapy drug candidate
By Bethany Halford


Flow chemistry has been steadily gaining ground in academia in recent years, and in 2017 the continuous chemical synthesis method—in which tubes and T-junction mixers replace the flasks and stir bars used for batch reactions—managed to make inroads in the pharmaceutical industry. One milestone: Chemists at Eli Lilly & Co. used continuous-flow chemistry as a safer, faster, and cheaper way to make the chemotherapy drug candidate prexasertib monolactate monohydrate.
The Lilly team set up eight continuous process steps to make the compound. One step used hydrazine, a component of rocket fuel, which would have been too dangerous to use in a batch process. But perhaps the most notable part of this synthesis is that the Lilly chemists linked the final stages in the continuous manufacturing process to quality-control systems to achieve current Good Manufacturing Practices (cGMPs) standards required by regulatory agencies for drug production (Science 2017, DOI: 10.1126/science.aan0745).


Kevin P. Cole, the report’s lead author, explained in June that the group decided to go for a flow approach because Lilly needed only 24 kg of the drug candidate. Making prexasertib in batch equipment would have required an extensive cleanup afterward because the compound is potent and cytotoxic, so any residue could potentially contaminate future reactions. The small flow setup the team used could be dedicated to making this single compound and then discarded, if necessary, at no great cost, Cole said. The success of using flow to make prexasertib prompted Lilly to build a continuous-flow facility in Kinsale, Ireland, which opened in October.
Flow chemistry was popular elsewhere this year. Flow chemistry company Snapdragon partnered with Pfizer to prepare the highly reactive reagent allenyllithium (shown below) en route to making an important drug intermediate. Snapdragon also formed a pact with Johnson Matthey, a leading supplier of bulk pharmaceutical chemicals, to collaborate on flow chemistry for drug manufacturing.


Big moves for little machines
Geared up after last year’s Nobel nod, researchers put rotors, drills, pulleys, and more into action
By Bethany Halford
Since the 1980s, chemists have been creating molecular machines—single molecules that resemble and function like motors, rotors, and even tiny cars. When three molecular machine pioneers won the 2016 Nobel Prize in Chemistry, the field gained a certain gravitas: The whirligig molecules being created were certified as more than mere chemical curiosities. It’s not a surprise that molecular machines continued to make headlines this year, including a souped-up motor-rotor combo, polymer pulleys designed to boost battery performance, and the world’s first nanocar race.


“It is fascinating to see the current acceleration in the field,” says Ben L. Feringa, one of the 2016 Nobel laureates. “The recent ingenious designs, ranging from molecular pumps to reshaping polymers, reflect how molecular machines will bring responsive and adaptive behavior, enabling numerous potential applications.” Here C&EN revisits some favorite molecular machine stories of 2017.
Nanocars zoomed into action
Move over, NASCAR. Nanocars have taken to the racing stage. Actually, real race car drivers probably won’t need to move at all, because these little racers are measured in nanometers, and their racetracks have to be viewed with scanning tunneling microscopes. In April, six international teams participated in the world’s first nanocar race, which resulted in a tie. Top honors went to the Nanoprix Team, led by Rice University’s James M. Tour and the University of Graz’s Leonhard Grill. Their Dipolar Racer featured low-adhesion molecular wheels, alkynyl axles, an aryl chassis, and dipolar functionalities in the nanocar’s front and rear. Top honors also went to the makers of the Swiss Nano Dragster, a triangular molecule designed to glide and helmed by the University of Basel’s Rémy Pawlak and Ernst Meyer.
Drilling holes in cells
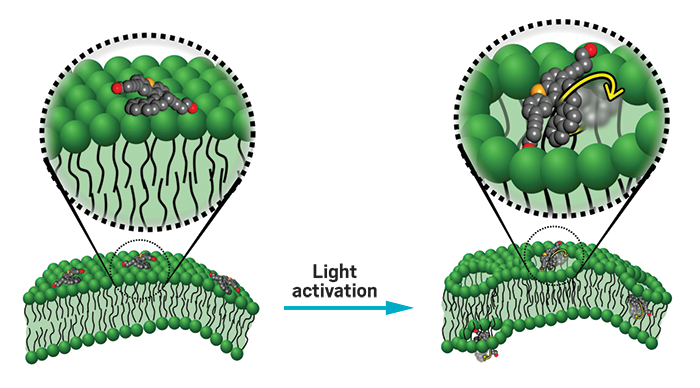

Scientists led by Rice University’s James M. Tour turned ultraviolet light-activated molecular motors into tiny drills that can bore through cellular membranes. The idea is to get enough motors drilling into cancer cells in order to destroy the cells’ integrity in a matter of minutes. Cancer cells can’t develop a resistance to the molecular motor’s motion the way they do for many chemotherapy treatments, Tour says (Nature 2017, DOI: 10.1038/nature23657).
Chiral construction by molecular machines
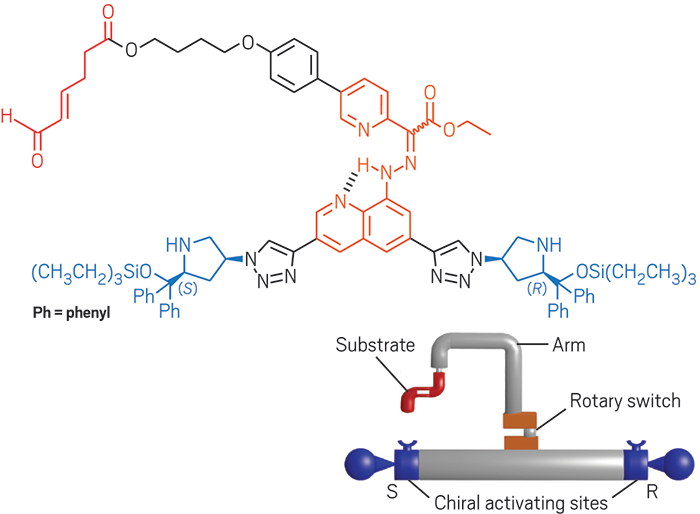

David A. Leigh and coworkers at the University of Manchester built a programmable molecular machine that creates four different products by adding thiol and alkene substituents asymmetrically to an α,β-unsaturated aldehyde substrate. The machine features an arm and rotatory switch that moves between a site for R stereochemistry and a site for S stereochemistry depending on pH (Nature 2017, DOI: 10.1038/nature23677).
Motor-rotor combo on the move
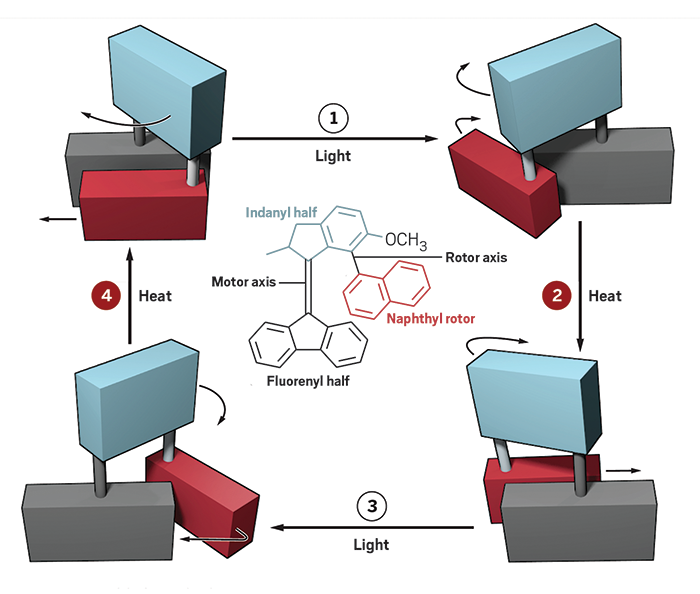

The University of Groningen’s Ben L. Feringa upped the complexity of the classic light-activated molecular motor his group is known for by attaching a rotor to it. The two components turn with synchronized movement thanks to the assembly’s complex stereochemical design (Science 2017, DOI: 10.1126/science.aam8808).
Winding and unwinding
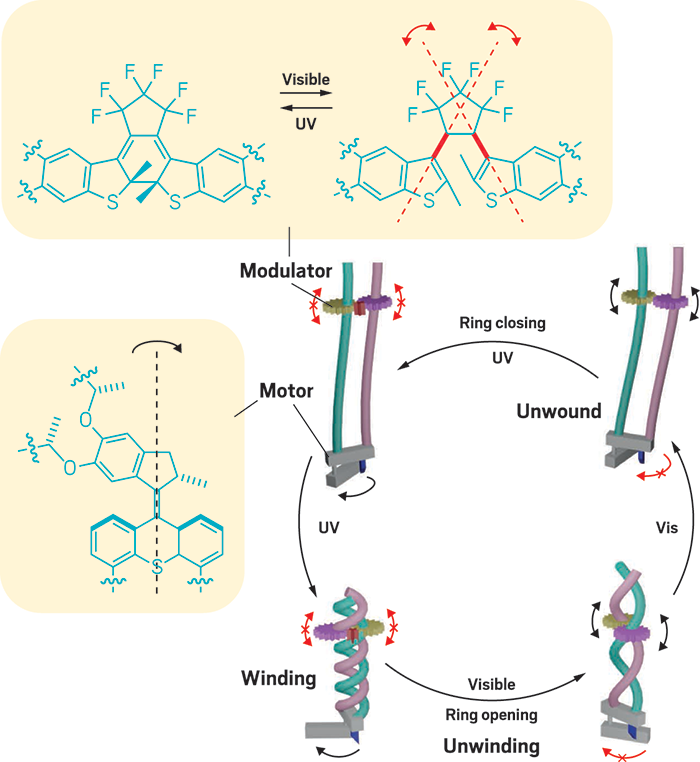

By combining two types of light-activated molecular machines, a team led by the University of Strasbourg’s Nicolas Giuseppone created a twisting system that winds and unwinds polymer chains, resulting in a material that contracts and expands depending on the wavelength of light shining on it. The system could lead to new types of actuators—for example, in artificial muscles (Nat. Nanotechnol. 2017, DOI: 10.1038/nnano.2017.28).
Polymer ‘pulleys’ for boosting battery performance
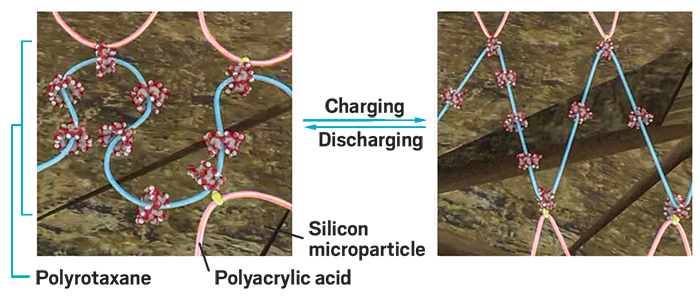

Researchers led by Ali Coskun and Jang Wook Choi at Korea Advanced Institute of Science & Technology developed polymers that, when added to a silicon anode, can relieve the stress the anode undergoes when charging and discharging so that it will last longer. The secret to the polymer’s success: a network made of the linear polymer polyacrylic acid covalently linked to polyrotaxanes containing mechanical bonds. During battery charging, as the silicon anode expands, the polyrotaxanes’ rings freely slide along the chain to dissipate stress, operating like a pulley system (Science 2017, DOI: 10.1126/science.aal4373).
Artificial devices mimicked the female reproductive system
A modular microfluidic device replicated the menstrual cycle and an artificial ovary restored fertility in mice
By Celia Arnaud

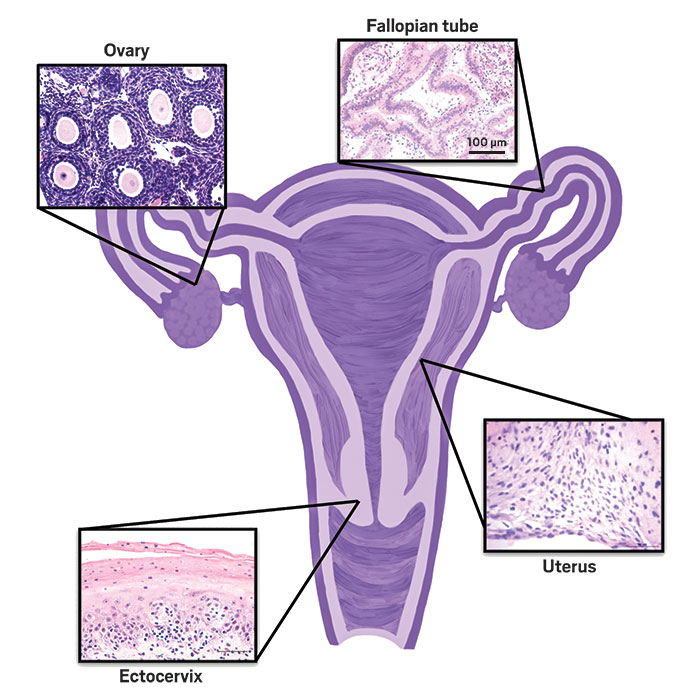
The female reproductive system is complicated. Its multiple organs, tissues, and hormones all have to work in concert to function properly. Imagine trying to replicate that in an artificial device. That’s what Teresa K. Woodruff of Northwestern University and coworkers did this year, reporting multiple advances in making engineered versions of this system.
First, Woodruff’s team reported a microfluidic device called the Evatar that reproduces the hormone profiles of the 28-day menstrual cycle (Nat. Commun. 2017, DOI: 10.1038/ncomms14584). Evatar contains modules with cultured tissue from mouse ovary and human fallopian tube, uterus, cervix, and liver connected by microfluidic channels and pumps. The researchers initiate the menstrual cycle by adding follicle-stimulating hormone. In response, the ovary module produces estrogen. At day 14, they add luteinizing hormone to trigger ovulation. In response, the ovary module stops producing estrogen and starts producing progesterone. The researchers need to add external hormones because those are normally produced by the pituitary gland, which is not included in the system.

Woodruff and her coworkers are receiving funding from the U.S. National Institutes of Health to adapt the technology to create models of diseases that affect reproductive-age women, such as polycystic ovary syndrome, fibroids, and endometriosis. They also have a grant from the Bill & Melinda Gates Foundation to further develop the technology to find new methods of contraception.
Woodruff doesn’t expect to stop at the reproductive system. Her team plans to add modules with other types of tissues to investigate how hormones such as estrogen affect other systems in the body. Currently the researchers have pancreas organoids producing insulin to provide on-device metabolism, and they are using testis and prostate cultures to study the toxicity of drugs on male testosterone production.
In other work, Woodruff teamed up with Ramille N. Shah of Northwestern to make artificial ovaries (Nat. Commun. 2017, DOI: 10.1038/ncomms15261). They seeded three-dimensionally printed gelatin scaffolds with ovarian follicles, which are the structures that house immature eggs. When the researchers implanted seeded scaffolds in mice whose ovaries had been surgically removed, the mice were able to mate and bear young naturally. Prosthetic ovaries incorporating human follicles are more challenging to build, Woodruff admits, but she sees them on the horizon.

Single-molecule experiment unveiled polymer growth spurts
Researchers got the first detailed look at how catalysts crank out polymer chains
By Steve Ritter


Cornell University researchers achieved the first real-time visualization of single polymer chain growth this year. What they saw came as a big surprise to them and the polymer research community. Using a pair of magnetic tweezers, optical microscopy, and spectroscopic techniques, the Cornell team discovered that individual polymer chains don’t grow smoothly and continuously from a catalyst as chemists had envisioned, but instead undergo consecutive wait and jump steps (Science 2017, DOI: 10.1126/science.aan6837).
The view of how polymer growth unfolds had been murky because of the limitations of analytical techniques. Researchers typically use methods such as dynamic light scattering to observe all the molecules in a polymer sample at once, then extract information about size distributions of the chains and other polymer parameters from the data. But those data are a challenge to interpret without corroborating spectroscopic methods.
The Cornell team led by Peng Chen, Geoffrey W. Coates, and Fernando A. Escobedo coupled its experimental approach with molecular dynamics computer simulations to get an unobscured view of polymer growth. The researchers attribute the jerky mechanism to formation of polymer tangles, which they call hair balls, that form around the catalyst as thousands of new monomer units are added to the growing chain. The hair balls randomly unravel after a couple of minutes, and a new hair ball starts to form.
Besides helping researchers better understand polymerization processes, the growth spurt discovery may be relevant to how cells produce proteins, nucleic acids, and polysaccharides, the team suggests.
“The ability to see dynamics in an important reaction like polymerization and to understand them through modeling is an exciting technological advance,” commented reaction dynamics expert Suzanne A. Blum of the University of California, Irvine.
“This is just a superb piece of science,” added polymer chemist Craig J. Hawker of the University of California, Santa Barbara. “The Cornell team’s tantalizing view of how polymer chains grow sheds light on many synthetic challenges dating from the earliest days of polymer chemistry.”
The Cornell researchers continue to study polymer hair-ball formation to understand its properties in more depth and are pushing their measurements toward single-monomer resolution, Chen said.
Probiotics found success in a large trial
Combination of probiotic strain and prebiotic sugar prevented sepsis in trial involving 4,556 infants
By Michael Torrice


Scientists continue to uncover ways in which microbes living in our bodies interact with and influence our biology. And some researchers hope to apply that growing knowledge to develop probiotic bacteria to treat or prevent disease.
This year, scientists reported a major demonstration of the probiotic field’s promise. In a trial involving more than 4,500 infants in rural India, a probiotic bacterium combined with a prebiotic sugar prevented sepsis, a potentially fatal inflammatory condition triggered by a bacterial infection in the blood (Nature 2017, DOI: 10.1038/nature23480).
Study leader Pinaki Panigrahi of the University of Nebraska Medical Center thinks the combination, called a synbiotic, could provide an inexpensive way to help tackle early-infant mortality in the developing world. In the multiyear trial, the synbiotic reduced cases of sepsis and death by 40%. The results were so good that the board overseeing the trial ended it early because it was no longer ethical to give infants the placebo.
These are landmark results, says Barbara B. Warner, who studies the neonatal microbiome at Washington University School of Medicine in St. Louis. She thinks one of the major lessons from the study is that probiotic researchers need to do their homework to find bacterial strains that will stably colonize the gut.
Before the trial began, Panigrahi’s team screened more than 200 strains of bacteria in cell culture and in rabbit studies to find microbes that could adhere to the intestines and block harmful bacteria from attaching to and slipping out of the gut and into the bloodstream. They also found that the prebiotic sugar fructooligosaccharide helps the optimal strain, Lactobacillus plantarum, colonize the gut. This kind of preliminary research on probiotic strains “is not frequently seen in the probiotic literature,” Warner says.
The study reinforces the idea that we can design ways to modify our gut microbiomes, Warner adds. “It’s a great piece of science to highlight, and I hope that we all learn from it.”
